I was just at the Heart Rhythm Society meeting in New Orleans, LA where there was a lot of very interesting health technology, digital health and artificial intelligence (AI) tools being discussed and on display. One such technology that I think would be of interest to readers of this site was Volta Medical.
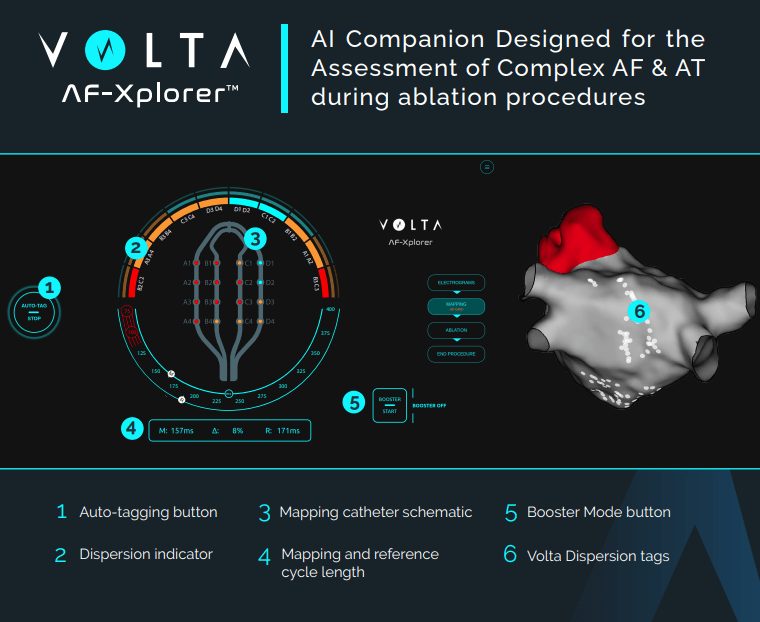
Volta Medical is a health tech company that develops AI software solutions to assist cardiac electrophysiologists during catheter ablation. They are currently most focused on atrial fibrillation (AF), and their product (computer terminal and software that connects to standard EP mapping systems) is known as AF-Xplorer (also referred to in previous reporting and clinical data as VX1). AF-Xplorer (AFX) is a cue-giving interface that helps identify abnormal electrograms that display spatiotemporal dispersion and allow those locations to be tagged on electroanatomical maps that can then guide ablation. Tagging can now occur automatically if using the Abbott Ensite X system, or via active participation of the clinical mapping specialist for other compatible systems like Biosense Webster Carto. This software received FDA approval in 2020 under regulatory class 21 C.F.R. § 870.1425 as a programmable diagnostic computer. There is not a lot of information about the specifics of their technology, but from the data available, we know that it consists of supervised machine leanring models (presumably neural networks, but this is unclear) trained and validated on a proprietary database of over 250,000 EGMs which were labeled by two operators. By pre-training their algorithms on this labled dataset, the AFX software has learned to recognize and annotate spatiotemporal dispersion in real-time.
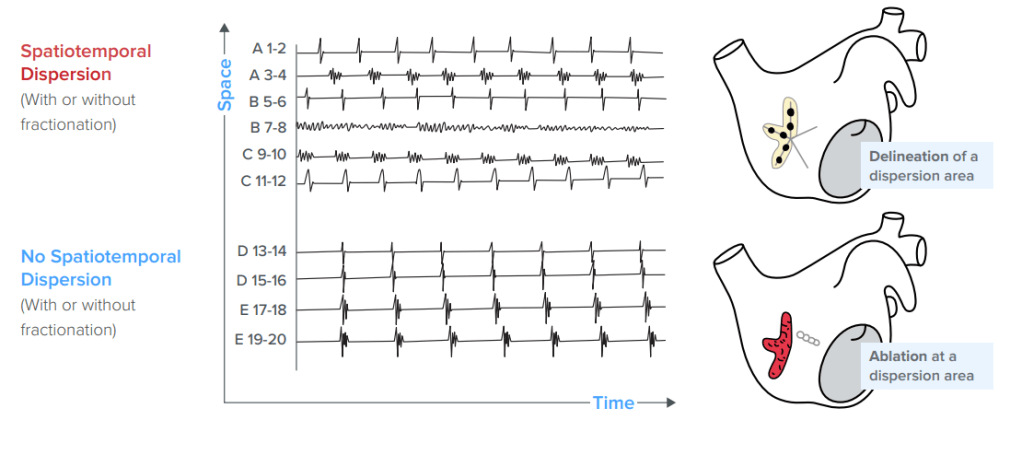
Spatiotemporal dispersion (STD) is defined as clusters of electrograms (EGMs) that display time and space dispersion (over a minimum of 3 adjacent electrodes) so that electrical activation is seen throughout the AF cycle length. These signals can be fractionated or not. Early evidence supporting the ablation of regions with EGMs that display STD was reported in this study. It was a non-randomized trial that enrolled 105 patients with drug-refractory AF (both paroxysmal and persistent) from 3 hospitals in France. It compared ablation of STD only to a control group that received standard pulmonary vein isolation +/- linear lesions and operator chosen EGM-based lesions. The endpoint was termination of AF during the ablation procedure. Average age of the study group was 63 years (+/- 11 years), 24 patients had paroxysmal AF and 81 were persistent or long-standing. Of the 105 patients, 100 (95%) achieved termination of AF from ablation during the procedure (either to sinus rhythm or atrial tachycardia). This compares to just over 60% conversion rate in the comparator group. They also looked at long-term results, which were defined as freedom from atrial arrhythmia over 18 months of follow-up. For a single procedure, freedom for arrhythmia was 55% vs 36% and for multiple procedures (average 1.4) was 85% vs 59%. Recurrences were assessed in any patient with symptoms during follow-up and via 24 hour Holter monitors in all patients at 3, 6, 12 and 18 months.
The above results were part of the impetus for developing AI that could standardize the identification of EGM STD. The VX1 software was tested in the Ev-AIFib trial and results were released last year. This trial was non-randomized and enrolled 85 patients with persistent and long-standing persistent AF from 8 hospitals in France and evaluated the technology in the hands of 17 different operators. Operators were instructed to first ablate all areas of tagged dispersion, and could include a PVI at their own discretion. Primary analysis looked at rates of conversion to sinus rhythm during ablation as well as long-term (13 months) freedom from AF and other atrial arrhythmias. Ablation led to acute termination of AF in 88% percent of patients in the VX1 group. 13 month freedom from AF after 1 procedure was 86% and freedom from any atrial arrhythmia was 73% (mean of 1.3 procedures). The key takeaways from this study addressed by the company were (i) validation of their AI tech, (ii) reproducibility of results in the hands of multiple operators, i.e. not just those with experience identifying and ablating STD, and (iii) above standard results at 12 months in a population with persistent or long-standing persistent AF.
There have certainly been other non-randomized studies showing promising results for substrate targeting with catheter ablation in persistent AF. Unfortunately, those early results have often failed to be verified in larger randomized clinical studies. Importantly, Volta Medical is testing this technology in a large, international, multicenter, randomized trial (Tailored-AF, NCT04702451) which recently finished enrollment. The primary endpoint of this trial is freedom from documented AF (lasting greater than 30 seconds) without an antiarrhythmic drug 12 months after a single procedure. Keep an eye out for those results – showing that AI can assist with standardization and successful ablation of the difficult quandary that is persistent AF would be a major win.
Please subscribe to our Newsletter for email notifications containing new posts as soon as they are published:
