As reported by Wearable, Samsung has filed a patent for a system that enables continuous electrocardiogram (ECG) monitoring of arrhythmias such as atrial fibrillation, using its Galaxy smartwatches. Although a number of smartwatches (Samsung, Apple, FitBit, Withings) already allow users to record single lead ECG signals, they currently require users to manually initiate the ECG recording via contact with two electrodes. This requires active participation by the user and generally records only a short 30 second signal. The new system proposes translating photoplethysmography (PPG) waveforms—commonly used for heart rate monitoring—into ECG signals through a dynamic generative AI model. This approach would allow for automatic, continuous detection of irregular heart rhythms without user intervention and Samsung hopes it will enhance the monitoring accuracy and utility of its wearable devices.
This technology was originally reported in a research paper posted on arXiv. Please read the full post below for a detailed review of the research and technology.
Introduction
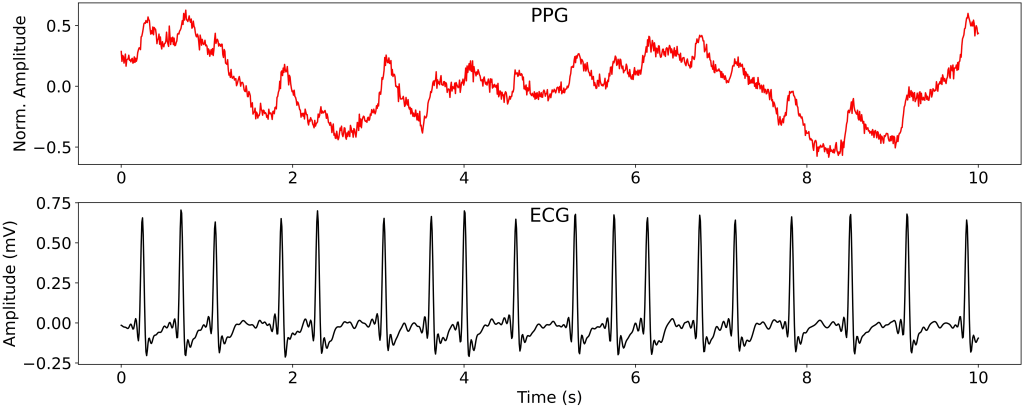
Atrial fibrillation (AF) is one of the most common cardiac arrhythmias, with significant implications for morbidity, mortality, and healthcare costs worldwide. Early detection is key to preventing complications like stroke and heart failure. Traditionally, ECG is the gold standard for diagnosing AF. However, ECG’s dependence on electrodes limits its practicality for continuous, real-world monitoring, a gap that PPG technology, found in many wearable devices, seeks to bridge. PPG measures heart rate using light absorption changes associated with varying blood volume, enabling non-invasive monitoring of cardiac rhythm activity. Despite widespread adoption, clinical utility has been constrained due to signal quality and inherent limitations in tracking complex cardiac patterns.
This recent study by Vo et al. introduces a novel deep state-space model leveraging an attention mechanism to translate PPG signals into ECG outputs, meant to enhance continuous, non-invasive AF detection. This approach, detailed in the referenced paper “PPG-to-ECG Signal Translation for Continuous Atrial Fibrillation Detection via Attention-based Deep State-Space Modeling,” represents a pioneering step in advancing wearable health technology toward reliable cardiac monitoring. Let’s explore the methodology, strengths, limitations, and potential clinical relevance of this innovative approach.
Methodology: Translating PPG to ECG with Attention-Based Deep State-Space Modeling
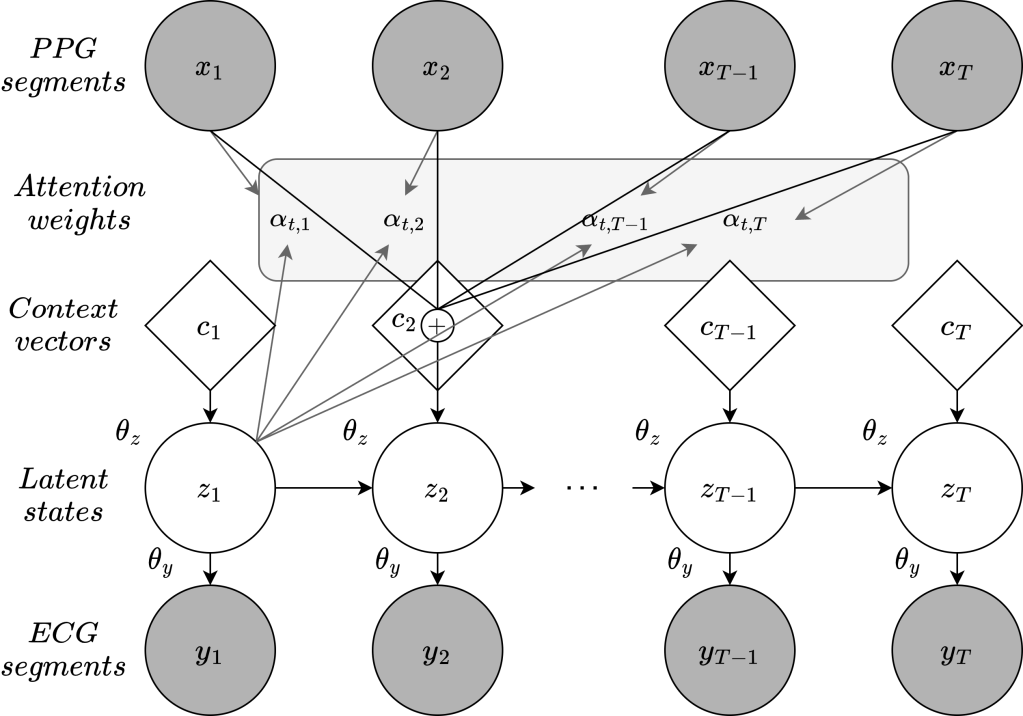
The model proposed by the authors addresses the fundamental challenge of translating PPG into ECG data using a two-layered, attention-based deep state-space model. This model is designed to process PPG data, translate it into a more diagnostically relevant ECG format, and enable accurate AF detection. Here’s a breakdown of its core components:
- State-Space Modeling: The model leverages a state-space architecture in which PPG signals serve as observable input data, while latent state variables represent the continuous ECG output. The use of a state-space model is crucial here as it helps manage the time-series complexities in both PPG and ECG, allowing for the seamless transition from a PPG waveform to an ECG-like representation.
- Attention Mechanism: Unlike conventional models, this approach integrates an attention mechanism that improves the model’s ability to align PPG signal segments with corresponding ECG outputs. This alignment is particularly important as PPG and ECG cycles are not inherently synchronized, and precise signal matching is essential for accurate reconstruction. The attention layer offers a context-aware perspective, enabling the model to recognize and prioritize relevant PPG features that correlate with ECG events.
- Probabilistic Approach for Noise Resilience: The authors designed the model with a probabilistic framework that enhances its noise resilience, which is especially valuable for real-world applications where PPG data is often compromised by motion artifacts, baseline drift, and other environmental interferences. They conducted tests simulating real-world conditions, including Gaussian and baseline noise, with the model demonstrating significant robustness.
- Model Evaluation and Validation: The authors trained and validated their model on the well-established MIMIC-III database, which contains detailed physiological data, including ECG records, from intensive care patients. The model achieved an impressive area under the precision-recall curve (PR-AUC) of 0.986 for AF detection, indicating high accuracy and a low rate of false positives. This is a promising result, particularly for continuous monitoring applications where false alarms can undermine user trust and clinical reliability.
Clinical Relevance
This model’s potential for clinical application is substantial, especially for managing AF or related arrhythmias in ambulatory and remote care settings. Continuous ECG-level arrhythmia detection would significantly enhance utility, allowing it to more readily identify a broad range of infrequent and asymptomatic arrhythmias (not just AF), support early detection, and improve risk stratification for certain patient groups. This technology could theoretically replace short-term Holter monitors, offering a more cost-effective, accessible tool for extended monitoring that aligns with current user-centric digital health trends. Challenges such as data management, privacy and regulatory approval would need to be addressed to realize this application’s full potential in clinical care.
Strengths of the Approach
- Scalable Continuous Monitoring: This model’s use of PPG data, readily available in consumer-grade wearable devices, paves the way for scalable, non-invasive, and continuous AF monitoring. By transforming PPG into an ECG format, the model enhances diagnostic value without requiring users to wear electrodes or complex equipment.
- High Noise Resilience: The robustness of the model against noise, achieved through its probabilistic design, is a critical advantage. Motion artifacts, ambient light fluctuations, and baseline drift are common in real-world wearable data. The model’s capacity to handle these interferences increases its reliability and diagnostic value.
- Computational Efficiency: Despite its complex architecture, the model demonstrates computational efficiency, partly due to the state-space approach and attention mechanisms that streamline processing. This efficiency is crucial for wearable devices with limited battery life and processing power, making it feasible to implement on consumer-grade devices.
- Potential for Broader Arrhythmia Detection: While the study focuses on AF detection, the model’s approach could potentially be adapted to detect other arrhythmias, such as ventricular tachycardia or supraventricular tachycardia, broadening its clinical applications. Continuous ECG monitoring could enable comprehensive cardiac surveillance for patients with various rhythm disorders.
Limitations and Future Considerations
- Dependence on High-Quality PPG Signals: The model’s performance, while robust to some noise types, is contingent on the quality of PPG input. Inconsistencies in signal acquisition due to poor device fit, skin pigmentation, or ambient lighting conditions could limit the model’s accuracy. Ensuring consistent PPG quality across different device types remains an important consideration for broader application.
- Limited Validation Sample: While the MIMIC-III database provides a solid foundation for model training and initial validation, its cohort is limited (55 subjects, only 12 with AF) and may not fully represent the population diversity required for generalizable AF detection. Broader validation studies, including different demographics, device types, and clinical settings, will be crucial to confirm the model’s efficacy across diverse populations.
- Battery and Processing Constraints for Wearable Integration: Implementing this model on consumer wearables could strain battery life and processing power due to the attention-based architecture. Wearable technology advances, such as improved battery efficiency and on-device processing capabilities, will be needed to enable long-term, continuous monitoring without frequent recharging or data offloading.
- Regulatory and Clinical Approval Pathways: For this model to be used clinically, it would need to go through rigorous validation and regulatory approval processes, including FDA clearance. Demonstrating clinical efficacy, safety, and reliability will be essential for acceptance by both healthcare providers and regulatory bodies.
Conclusion
The attention-based deep state-space model for translating PPG signals to ECG outputs represents a promising leap forward in wearable-based cardiac monitoring. This approach has the potential to provide clinicians with continuous, reliable AF detection outside traditional healthcare devices, supporting early intervention and preventative care strategies. While challenges remain, this model showcases how advanced AI techniques can enhance the diagnostic capabilities of consumer-grade health technologies.
Please subscribe to our Newsletter for email notifications containing new posts as soon as they are published:
